Introduktion af Spectrum
Det elektromagnetiske spektrum er en fascinerende række af bølger, der definerer alt fra radiosignaler til synligt lys. Inden for dette spektrum bestemmes de farver, vi ser, af deres bølgelængder, og hver farve indtager en bestemt position i spektret. Forståelsen af forholdet mellem bølgelængder og farver uddyber ikke kun vores forståelse af naturfænomener, men afslører også praktiske anvendelser inden for teknologi og hverdagsliv. Denne artikel dykker ned i den spændende verden af farver med korte bølgelængder, deres betydning og deres rolle i vores dagligdag.
Videnskaben om farver
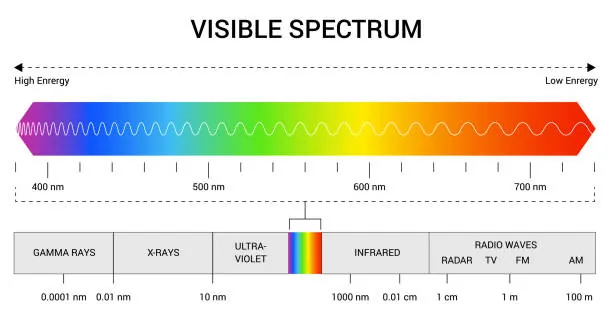
Farver er mere end bare visuelle elementer - de er et direkte resultat af samspillet mellem lys og det menneskelige øje. Lyset bevæger sig i bølger, og bølgelængden af disse bølger bestemmer den farve, som vores hjerner opfatter. Det synlige spektrum spænder fra ca. 380 til 700 nanometer og omfatter alle de farver, vi kan se. Kortere bølgelængder svarer til farver som violet og blå, mens længere bølgelængder resulterer i røde og orange farver.
Forholdet mellem bølgelængde og farve har rødder i fysikken. Når lys rammer et objekt, absorberes visse bølgelængder, og andre reflekteres. Det reflekterede lys kommer ind i øjet, hvor specialiserede celler kaldet tappe fortolker det som en bestemt farve. Denne opfattelse gør forståelsen af bølgelængder afgørende for at udforske den livlige og varierede verden omkring os.
Farver med kort bølgelængde
I det synlige spektrum omfatter farver med kortere bølgelængder violet, indigo og blå. Violet har den korteste bølgelængde, der måler omkring 380 til 450 nanometer, tæt fulgt af blå, der spænder over 450 til 495 nanometer. Disse farver har deres karakteristiske udseende på grund af deres høje energiniveauer og kortere bølgelængder.
Grunden til, at violet og blå ser ud, som de gør, ligger i deres interaktion med lys. Farver med kort bølgelængde spredes mere effektivt, hvilket er grunden til, at himlen ser blå ud om dagen. Selvom violet har en kortere bølgelængde end blå, er den mindre synlig for det menneskelige øje, fordi vores øjne er mere følsomme over for blåt lys.
Der er masser af eksempler på farver med korte bølgelængder i naturen. Den livlige blå farve på en klar himmel, de fascinerende nuancer i en safir og de dybe purpurfarver i nogle blomster viser skønheden i disse bølgelængder. De betager vores sanser og minder os om det komplicerede forhold mellem lys og farve.
Anvendelser af farver med kort bølgelængde
Farver med kort bølgelængde har en bred vifte af anvendelsesmuligheder inden for teknologi, design og psykologi. Inden for teknologi spiller blåt lys en afgørende rolle i enheder som LED-skærme, smartphones og moderne belysningssystemer. Violet lys i form af ultraviolette (UV) stråler har vigtige anvendelser inden for sterilisering og retsmedicin.
Disse farver har også en dybtgående indvirkning på humør og opfattelse. Blå forbindes ofte med ro og fokus, hvilket gør den til et populært valg til arbejdsområder og afslapningsområder. Violet fremkalder på den anden side kreativitet og spiritualitet og bruges ofte i miljøer, hvor inspiration er afgørende.
Desuden udnytter brancher som mode, kunst og indretning kortbølgede farver for deres dristige og fængslende kvaliteter. Deres alsidighed sikrer deres fortsatte relevans inden for både æstetiske og funktionelle områder.
Almindelige misforståelser om farvebølgelængder
På trods af deres videnskabelige grundlag bliver farvebølgelængder ofte misforstået. En almindelig myte er, at blåt lys i sig selv er skadeligt for øjnene. Mens overdreven eksponering for blåt lys fra skærme kan bidrage til digital øjenbelastning, er det ikke bølgelængden i sig selv, men intensiteten og varigheden af eksponeringen, der udgør en risiko.
En anden misforståelse er, at violet lys er usynligt. Selv om det er tættere på det ultraviolette område, er violet stadig en del af det synlige spektrum og kan opfattes, om end mindre tydeligt end blåt.
At afklare disse myter hjælper med at værdsætte videnskaben bag farver, samtidig med at den ubegrundede frygt for deres virkninger imødegås.
Konklusion af Bølgelængdefarver
Udforskningen af farver med kort bølgelængde - violet, blå og indigo - afslører deres videnskabelige, praktiske og æstetiske betydning. Disse farver, der er forankret i principperne for det elektromagnetiske spektrum, former vores oplevelser på utallige måder, fra naturens skønhed til den teknologi, vi bruger dagligt.
Forståelsen af farvernes bølgelængder beriger vores opfattelse af verden og fremhæver deres dybe indflydelse på vores liv. Efterhånden som videnskaben og teknologien fortsætter med at udvikle sig, vil anvendelsen og forståelsen af farver med kort bølgelængde utvivlsomt blive udvidet og minde os om det synlige spektrums uendelige vidundere.
Almindelige spørgsmål
1. Hvordan har forskellige farver forskellige bølgelængder?
Farver har forskellige bølgelængder, fordi de reflekterer forskellige mængder af lysenergi. For eksempel bærer kortere bølgelængder som violet mere energi end længere bølgelængder som rød.
2. Hvorfor ser farver med korte bølgelængder lysere ud end farver med længere bølgelængder?
Farver med korte bølgelængder, som blå og violet, spreder lyset mere effektivt, hvilket får dem til at se lysere ud under bestemte forhold som f.eks. en solrig himmel.
3. Er farver med korte bølgelængder skadelige for øjnene?
Farver med korte bølgelængder er ikke i sig selv skadelige. Men langvarig udsættelse for intenst blåt lys, f.eks. fra digitale skærme, kan føre til overanstrengelse af øjnene, hvilket understreger behovet for mådehold og beskyttelsesforanstaltninger.
