Introduction of Visible Light Spectrum
The visible light spectrum is a small yet significant part of the electromagnetic spectrum, encompassing the colors we perceive with our eyes. Each color within this spectrum has a distinct wavelength and frequency, defining its energy and impact. Frequency, the number of wave cycles per second, is crucial to understanding how we perceive different colors and their unique properties. This article delves into the concept of visible light, exploring which wavelength has the highest frequency and the real-world applications of high-frequency light waves.
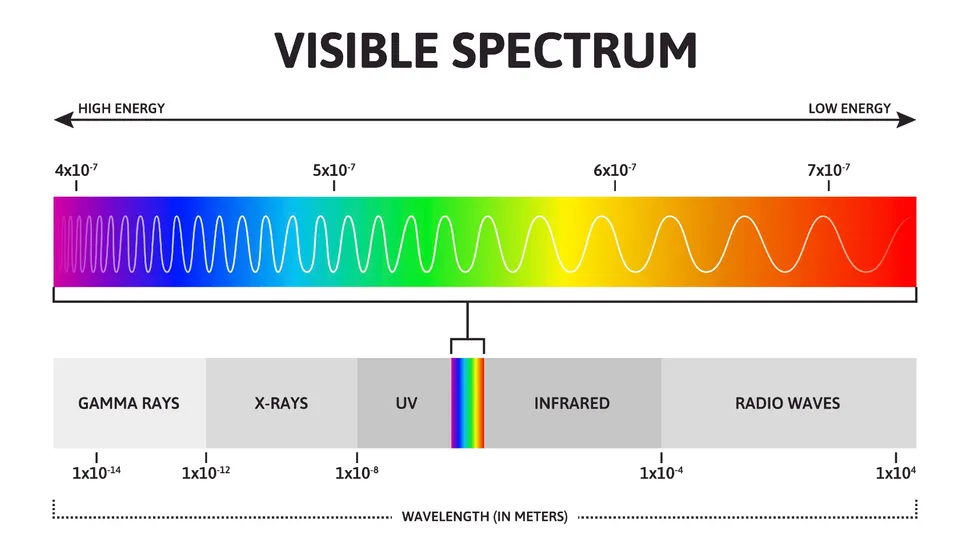
What is the Visible Light Spectrum?
Visible light ranges from approximately 380 to 700 nanometers (nm) in wavelength and represents only a fraction of the electromagnetic spectrum. Within this range, each color of light has its specific wavelength, from red with the longest wavelength to violet with the shortest. The colors of the visible spectrum in order are red, orange, yellow, green, blue, indigo, and violet (often remembered by the acronym ROYGBIV).
These colors represent various energies, with red having lower energy due to its longer wavelength and violet having higher energy because of its shorter wavelength. This spectrum plays a vital role in everything from the colors we see in nature to advanced technologies in science and industry.
Understanding Frequency in Light Waves
Frequency in the context of light waves refers to the number of cycles a wave completes in one second, measured in Hertz (Hz). There’s an inverse relationship between wavelength and frequency: as wavelength decreases, frequency increases. This is significant because a higher frequency means greater energy in a wave.
In the visible light spectrum, this relationship means that violet light, with the shortest wavelength, has the highest frequency and, therefore, the highest energy. This energy difference affects how colors are perceived, with higher-frequency waves appearing cooler and more intense in color than lower-frequency ones.
Determining the Highest Frequency Wavelength
To calculate the frequency (ff) of light, one can use the formula:
f=cλf = frac{c}{lambda}
where:
- ff = frequency,
- cc = speed of light (approximately 3.00×1083.00 times 10^8 meters per second),
- λlambda = wavelength.
Given that violet light has the shortest wavelength (around 380 nm), it possesses the highest frequency within the visible spectrum. Using the formula, we can see that the shorter the wavelength, the higher the frequency, confirming violet as the color with the highest frequency.
Applications of High-Frequency Light Waves
High-frequency light waves, especially those in the violet or near-ultraviolet range, have a range of practical applications due to their high energy. Some of the notable uses include:
- Medical Imaging and Treatments: High-frequency light waves play an essential role in ultraviolet therapies for skin treatments and in certain types of medical imaging that require more detailed visualizations.
- Forensic Science: High-energy ultraviolet light helps reveal substances that are otherwise invisible to the human eye, making it valuable in forensic investigations.
- Sanitization and Disinfection: High-frequency light, particularly in the UV-C range, is used for sterilizing medical equipment, water, and air by eliminating harmful bacteria and viruses.
- Optical Data Storage: High-frequency light, with its shorter wavelength, allows for the storage of more data on optical discs like Blu-ray, compared to lower-frequency red light used in older technologies like DVDs.
- Telecommunications: In fiber optics, high-frequency light waves allow for faster data transmission, increasing the efficiency and speed of communication systems.
These applications demonstrate the value of high-frequency light waves in advancing technology and enhancing everyday processes.
Common Questions about Frequency in the Visible Light Spectrum
1. What is the relationship between frequency and energy in light waves?
The relationship between frequency and energy is directly proportional: the higher the frequency, the greater the energy of the light wave. This is why violet light, with its high frequency, carries more energy than red light, which has a lower frequency.
2. How does the frequency of light waves affect human perception of color?
Higher frequency light waves are perceived as cooler colors, such as blue and violet, while lower frequencies appear as warmer colors, like red and orange. Our perception is influenced by both the frequency of the light and how it interacts with the rods and cones in our eyes, which detect different wavelengths.
3. Can different materials affect the frequency of light waves?
When light passes through different materials, its speed and wavelength may change, but its frequency remains constant. This phenomenon explains why a prism can split white light into its constituent colors by bending each wavelength to a different degree without altering their frequencies.
Conclusion
In summary, the visible light spectrum is an essential part of our perception, containing various wavelengths and frequencies that define color and energy. Among visible wavelengths, violet light has the highest frequency due to its shorter wavelength, which endows it with greater energy compared to other colors in the spectrum. High-frequency light waves have profound applications in fields ranging from healthcare and forensics to data storage and telecommunications. Understanding these properties of light enriches our appreciation of its role in both natural phenomena and technological advancements. The high-frequency end of the spectrum, especially violet, showcases the power of light in both visible beauty and practical applications that shape the modern world.
